Published on: June 11, 2022
Written by Liam Jaxon / Fact-checked by David Rowan
Life without batteries would be like living by steam or clockwork in a century or two. Tiny batteries power cell phones and laptops to fit in our pockets or carry on our backs, and we need them to live our contemporary lifestyles. First, we need to understand electrolytes and electron affinity to grasp the battery principle. There will be a potential difference between two dissimilar metals or metallic compounds immersed in an electrolyte, and a potential difference causes the current flow.

Depending on their electron affinities, two metals or metallic compounds gain or lose electrons in an electrolyte solution. So, it’s critical to understand the basic principles of batteries, whether rechargeable vs non-rechargeable AA lithium batteries, mobile batteries or car batteries. If you’re interested in learning more about how a battery works in-depth, you can check out my overall pick in this article.
What Is a Battery?
We all know different batteries from our childhood, but do we know what a battery explains? A battery converts chemical energy to electricity. An external circuit in a battery moves electrons from one substance (electrode) to another. A battery is made up of electrons moving around. An electric battery, unlike regular electricity, releases energy slowly over days, weeks, months, or even years.
People have always made energy on the fly. Prehistoric humans learned how to make energy (heat) from wood chemicals. The historical 1800s perfected our coal-burning skills. Making a fire can take an hour, and steaming up a locomotive’s boiler can take hours. On the other hand, Batteries deliver quick energy.
Types of Battery
Batteries vary in size, voltage, and capacity. There are two sorts of batteries: primary and secondary. Primary batteries are disposable and cannot be refilled; secondary batteries can be recharged several times, often hundreds. Following are the details:
Primary Batteries
You may think single-use disposable batteries are outdated and costly. Despite this, they have a major advantage: they store and last far longer than equivalent rechargeables. Some things that matter like heart pacemakers, which use surgically implanted, disposable lithium batteries. It’s simply not feasible to continually cut someone’s chest open to recharge their pacemakers!
In short, while it’s recommended to use rechargeable batteries wherever possible, disposable batteries have some advantages. Zinc-carbon, alkaline, and lithium are the three most common primary batteries.
Zinc-carbon
The cheapest, most common batteries for flashlights are zinc-carbon. A carbon rod is used for the positive electrode, which is encased in powdered carbon and manganese (IV) oxide; a zinc alloy is used for the negative electrode (outer casing).
The electrolyte is an ammonium chloride paste. When a zinc-carbon battery is connected, the two electrodes behave differently. The negative electrode converts zinc into zinc ions and electrons that power the circuit. From manganese (IV) oxide to ammonia at the positive electrode.
Alkaline
Alkaline batteries are more expensive than zinc carbon batteries because they store more energy and last longer. They are a particularly reliable source of power because they keep charged for years at a time. These chemicals are distinct from those used in zinc-carbon ones.
The positive electrode is manganese oxide; the negative electrode is zinc; the electrolyte is the solid alkaline solution (potassium hydroxide). Two chemical reactions provide power. Hydroxyl ions are produced from manganese (IV) oxide at the positive electrode. Activating the circuit requires zinc to react with the hydroxyl ions.
Button Cells (Lithium or Zinc)
Others employ lithium and organic electrolytes and work through other chemical processes. In a button cell, the top centerpiece is the negative electrode, consisting of zinc or lithium. The positive electrode is constructed of manganese oxide, silver oxide, or copper oxide. The positive electrode in button batteries used to be mercury oxide and graphite; however, mercury is hazardous and has been removed from batteries.
Secondary Batteries (Rechargeable)
It’s more customary to talk of rechargeable than “secondary” batteries. Comparatively, a rechargeable battery is a battery that can be charged, discharged into a load, and repeatedly recharged, as opposed to a disposable or primary battery. It contains electrochemical cells.
The word “accumulator” is used because it stores energy via an electrochemical reaction. Rechargeable batteries come in all shapes and sizes, from button cells to megawatt systems connected to stabilize an electrical distribution network. Lithium iron phosphate (LiFePO4) and lithium-ion polymer (LiIP) are among the electrode materials and electrolytes employed (Li-ion polymer).
Rechargeable batteries often cost more initially than disposable batteries but have a reduced total cost of ownership and environmental effect due to their multiple rechargeability. Some rechargeable batteries are interchangeable with disposable batteries in terms of size and voltage.
Lead-Acid Batteries
Lead-acid batteries have been around since the mid-19th century. Their total output is 12 volts, divided by six 2-volt cells. Each cell has a negative lead metal electrode, a positive lead dioxide electrode, and a sulfuric acid electrolyte. On discharge, lead sulfate coats both electrodes, sulfuric acid is mostly converted to water, and electrons flow out along the external circuit to create power.
Nickel-Cadmium:
Nickel-cadmium (NiCd) batteries are used in toys, flashlights, and power tools. They are affordable and can be charged and discharged hundreds of times. However, they must be fully discharged before charging. Otherwise, their storage capacity (and longevity) would be much diminished.
Whether or whether this is true, routinely draining and recharging batteries is suggested. The cadmium in NiCd batteries is also problematic, and cadmium can seep into the soil and potentially pollute nearby waterways instead of recycling them.
Nickel-metal-hydride (NiMH)
NiMH batteries perform similarly but suffer less from the “memory effect.” They became popular in the 1990s as a replacement for NiCd batteries due to cadmium concerns. Cellphones, for example, use NiMH batteries that operate better when “topped up” rather than fully discharged and recharged (which is more typical with something like power tools).
Lithium-ion
Lithium-ion batteries are the most popular rechargeable batteries widely used in cellphones, MP3 players, and laptops. It’s a light metal that generates ions quickly, making it ideal for batteries.
Unlike NiCd rechargeables, lithium-ion batteries store more energy, perform at higher voltages, and are more ecologically friendly. These batteries are perfect for everyday usage in electrical devices that aren’t supposed to last that long and maybe charged and drained hundreds of times.
What Are The Battery’s Primary Components?
It is essential to know what is the basic principle of a primary cell if you want to know the details of a battery. A battery has three parts: electrodes, electrolytes, and separator. Typically, every battery has two electrodes, and their conductive materials fulfill distinct functions.
This is where the cathode connects to the positive end of the battery and where the current leaves (or electrons enter) the battery when it is discharged. These items are normally protected by a metal or plastic outer shell for our convenience and security.
External electrodes are connected to two more handy electrical connections marked with a plus (+) and minus (-). A battery comprises two or more cells with their power added together, making a difference.
The anode links to the battery’s negative end and is where current enters (or electrons leave) the battery during discharge. The separator is the final component of the battery and keeps the anode and cathode separated inside the battery. Devoid a separator, the two electrodes would short-circuit and render the battery useless.
How a Battery Works
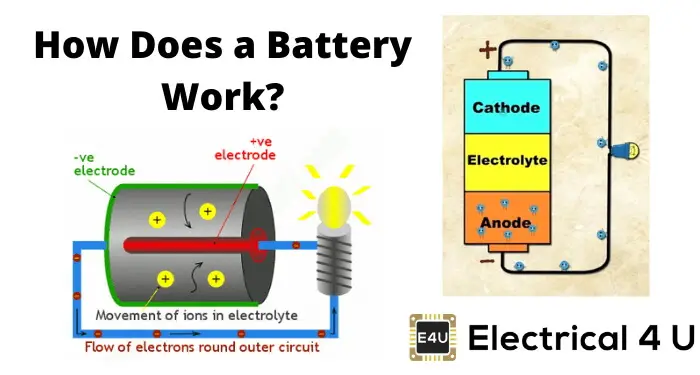
To understand the basic principles of batteries, you need to know how they work. Imagine putting double AA alkaline batteries in a flashlight to understand a battery. When you put the batteries in the flashlight and turn it on, you’re completing a circuit. The chemical energy in the battery is converted to electrical energy and flows into the base of the flashlight’s bulb, causing it to glow. The electric current then re-enters the battery, but from the other end.
The electrodes in a battery include atoms of specific conducting materials. In an alkaline battery, the anode is zinc, while the cathode is manganese dioxide. The electrolyte between and inside such electrodes includes ions, and Electrochemical processes occur when these ions come into contact with the electrodes’ atoms.
The electrodes’ oxidation-reduction reactions (redox reactions) are collectively known as redox reactions. The cathode receives electrons from the anode, making it the oxidizing agent in batteries, and the anode is the reducing agent because it loses electrons. Ultimately, these reactions result in ion movement between the anode and cathode and electron release from electrode atoms.
These liberated electrons concentrate inside the anode (the bottom, flat part of an alkaline battery). The anode becomes negatively charged when electrons are released, whereas the cathode becomes positively charged as electrons are consumed. Due to the charge difference, the electrons are drawn to the positively charged cathode. They can’t get inside the battery since the separator blocks their access.
All that changes when you turn on your flashlight. The electrons can now reach the cathode. But first, they must pass through the bulb’s base. The circuit is completed when the current returns to the battery through the cathode.
How Can a Battery Be Damaged
A battery can be damaged by:
- Overcharging
- Overheating
- Over discharging
- Physical damage (e.g. puncturing, crushing)
- Using a charger with the wrong voltage or amperage
- Using the battery in extreme temperatures
- Using a non-compatible device or charger.
FAQs
What Is the Cell?
The cell is a single power source that stores chemical energy and transforms it into electrical energy. It has two electrodes: a cathode and anode. The cell contains an electrolyte, a chemical that reacts with the electrodes to produce electricity.
What Is Contained Within the Battery?
The typical alkaline AAA, AA, C, D, 9-volt, or button-cell battery is composed primarily of steel and a mixture of zinc, manganese, potassium, and graphite, with the remainder of the battery being composed of paper and plastic.
How Do Batteries Store Energy?
Like many other energy sources, batteries store energy using chemistry in chemical potential. For example, logs store energy in their chemical bonds until burned to produce heat. A battery requires an external circuit to collect and release energy.
How Do Batteries Hold a Charge?
Batteries hold a charge by using an electrochemical reaction to store energy as ions in a separator between two electrodes, a positive cathode and a negative anode. The separator allows ions to flow between the electrodes when the battery is in use, but prevents the electrodes from touching, which would short-circuit the battery. When the battery is charged, the electrodes absorb ions, and when the battery is discharging, the electrodes release ions. This process creates a flow of electricity that can be used to power devices.
What Are the Three Primary Electrolytes in the Battery?
Sodium, potassium, and chloride are the three primary electrolytes found in the battery.
How Does Electricity Flow in a Battery?
A positive charge would move in the same direction as an electric current. So the external circuit current flows away from the battery’s positive end and toward its negative terminal, and electrons would flow oppositely through the wires.
Final Thought
Batteries and similar devices accept, store, and discharge electricity. Like many other energy sources, batteries store energy using chemistry in chemical potential, and the primary battery principle is the storage of chemical energy and conversion to electrical energy.
Always remember batteries leak chemicals into soil, contaminating groundwater and surface water. So it needs to be properly disposed of; otherwise, the harmful compounds of batteries can harm human health and contribute to global warming.
About This Writer

Hi, I am responsible for the 'Homeowners Power Solutions' category. My name is Liam Jaxon and a licensed technician with 7 years of experience in vehicle batteries, electrical gadgets, and home appliances. My working experience in different residential & light commercial electrical sectors and the automobile industry helped to acquire vast knowledge in this industry.